How can the pharmaceutical industry use tools to make operations more compliant and what does integrity mean in the industry? Medicines and remedies can significantly influence patients’ lives. Safety, effectiveness and the pharmaceutical industry are key factors in regulating them. It is important to regulate medicines and remedies strictly. Pharmaceutical companies must follow the rules for making, testing and selling. Companies must adhere to the regulations when marketing drugs in clinical trials and operations. Regulatory compliance in pharmaceutical industry is not a choice, but a necessity. The impact of non-compliance can be severe, leading to substantial fines and legal entanglements.
Compliance in the pharmaceutical industry is important. Drug manufacturers must adhere to regulations and product approval and ensure patient access. This will reduce the risk of non-compliance and other threats in the industry.
This helps reduce the risk of non-compliance and other industry threats. It requires them to understand the regulatory bodies that oversee them. It also remains informed of new guidance and its applicability, as well as enforces regulatory compliance. With that let’s understand what it is, the challenges, best practices along with strategy in pharmaceutical industry. Let’s begin.
What is Regulatory Compliance in Pharmaceutical Industry?
Regulatory compliance refers to an organisation’s rules and regulations for its operations. Pharmaceutical manufacturers adhere to regulations and laws established by governing and regulatory bodies.
Adhering to regulatory standards is imperative rather than discretionary within the pharmaceutical sector. As a business owner, you ensure your pharmaceutical company follows all industry rules.
The pharmaceutical industry is subject to stringent regulations for many reasons. First, the sector focuses on the health and well-being of individuals.
Furthermore, healthcare is a significant financial burden for the majority of individuals. Governments and industry organisations have implemented stringent guidelines and regulations. It ensures that pharmaceuticals are marketed and sold ethically and responsibly.
Importance of Regulatory Compliance
Let’s delve into the main reasons why regulatory compliance in pharmaceutical industry is so vital:
1. Patient Safety
Pharmaceutical sales and marketing compliance regulations are not just about rules and regulations. They are about ensuring patient safety. By providing correct information, patients are empowered to discuss their health issues and use medications only as described, thereby staying safe.
This helps prevent patients from receiving the wrong medicine for their health issues. It also mitigates off-label promotion, misleading claims and hazards linked to inaccurate information.
2. Regulatory Adherence
Pharmaceutical companies operate within a complex network of regulations and guidelines. These processes are closely monitored by regulatory agencies such as the FDA, EMA and other authoritative bodies. Compliance with these regulations is crucial, as it demonstrates an organisation’s commitment to ethical practices and patient safety.
3. Protection against Misleading Practices
Compliance regulations protect against deceptive marketing practices. They ensure doctors and patients have accurate information to make informed decisions. They also help out in preventing false or exaggerated claims about medicine. Compliance guidelines regulate advertising content to ensure fairness and non-intensification of patients’ decision-making.
4. Trust and Reputation
Upholding pharmaceutical marketing and sales compliance is not just a legal requirement, it’s a commitment to maintaining the sector’s standing. By adhering to rules and standards set by regulators and ethics codes, organisations demonstrate their care for patients and ethical practices.
This enhances the organisation’s reputation and ensures sustained prosperity by improving confidence among healthcare practitioners, patients, regulatory bodies, and other interested parties.
Complying with regulations also exposes pharmaceutical companies to various risks, including the possibility of incurring legal penalties, fines, or prosecution. Reputational harm can cause large business losses, a decline in investor confidence, and disruptions to many pharmaceutical operations.
7 Common Compliance Challenges in Pharmaceutical Meetings
The pharmaceutical industry is vital to protecting public health. The industry conducts research, develops and produces life-saving medications. Because of the potential risk associated with the industry, production and distribution must follow strict rules and regulations.
Adhering to regulatory standards is critical to protect patient well-being. It also plays a crucial role in upholding the industry’s integrity. Here are the seven common compliance issues the pharmaceutical industry faces. Let’s dive into this in detail.
1. Good Manufacturing Practices (GMP) Violations
In India, adherence to Good Manufacturing Practices (GMPs) is crucial for ensuring the quality and safety of pharmaceutical products. However, the industry faces challenges related to GMP violations due to factors such as inadequate infrastructure, resource constraints and a large number of small-scale manufacturers.
Non-compliance with GMPs often results from insufficient training and awareness among personnel, leading to deviations from standard operating procedures and lapses in documentation. This poses significant risks to public health, as substandard or adulterated medications may enter the market, potentially causing harm to patients.
Additionally, GMP violations can damage the reputation of Indian pharmaceutical companies internationally, affecting their ability to export products and compete in global markets. Thus, addressing GMP compliance issues is essential for maintaining the integrity of the pharmaceutical sector in India and safeguarding patient well-being.
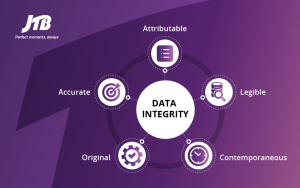
2. Data Integrity
Ensuring data integrity in the Indian pharmaceutical industry is paramount for maintaining the credibility of clinical trials, regulatory submissions and product quality. However, the sector grapples with challenges such as inadequate infrastructure for data management, limited regulatory oversight and the proliferation of counterfeit drugs.
These factors contribute to instances of data manipulation, unauthorized access and loss, compromising the accuracy and reliability of pharmaceutical records. Furthermore, the lack of stringent enforcement mechanisms exacerbates the problem, allowing unethical practices to persist. In India, where the pharmaceutical market is vast and diverse, ensuring data integrity requires concerted efforts from regulatory authorities, industry stakeholders and healthcare professionals.
Strengthening regulatory frameworks, investing in robust data management systems and enhancing awareness and training on data integrity principles are essential steps towards addressing this challenge and upholding the highest standards of transparency and accountability in the Indian pharmaceutical sector.
3. Adverse Event Reporting
Pharmaceutical companies must disclose any incidents of damage linked to their products. Compliance concerns ensue when organisations fail to detect and report adverse events during prescribed periods. Not reporting side effects harms patients and damages the pharmaceutical industry’s reputation.
In India, timely and accurate reporting of adverse events associated with pharmaceutical products is essential for safeguarding public health and ensuring regulatory compliance. However, the Indian pharmaceutical industry faces challenges in detecting and reporting adverse events due to factors such as underreporting, lack of standardized reporting mechanisms and insufficient pharmacovigilance infrastructure.
Moreover, cultural factors, such as hesitancy among healthcare professionals to report adverse events or concerns about potential repercussions, may further impede effective reporting practices. As a consequence, there is a risk of delayed or incomplete reporting, which can compromise patient safety and erode trust in the Indian pharmaceutical regulatory system.
4. Compliance in the Supply Chain
The pharmaceutical supply chain involves many parties, including pharmacies, distributors and manufacturers. Ensuring compliance with supply chain rules can be challenging. This is especially true when dealing with counterfeit drugs or improper pharmaceutical handling. Monitoring and enforcing adherence to regulations in these situations can be difficult.
Maintaining control over the supply chain becomes even more crucial in such cases. Non-compliance with the supply chain results in administering substandard or counterfeit medications to patients.
5. Fraud and Corruption
The pharmaceutical industry is at risk of fraud and corruption, including bribery, off-label promotion, and illegal kickbacks.
Fraud and corruption are serious issues within the industry. Unethical conduct may taint patient safety and obstruct fair competition. Non-compliance may also hinder a company’s reputation. It also causes financial losses and has legal repercussions.
Not only that but fraud and corruption pose significant challenges to the Indian pharmaceutical industry, threatening ethical standards, fair competition, and public health. Instances of bribery, off-label promotion, and illegal kickbacks are not uncommon, undermining the integrity of the industry and eroding trust in pharmaceutical products and healthcare professionals.
Furthermore, regulatory enforcement mechanisms in India may be inadequate or susceptible to manipulation, allowing unethical practices to persist. Addressing fraud and corruption requires a multi-faceted approach, including robust regulatory oversight, transparency in business practices, and efforts to promote ethical conduct among industry stakeholders.
6. Intellectual Property Protection
Protection of intellectual property (IP) is the foundation for innovation in the pharmaceutical industry. Compliance issues manifest when corporations neglect to safeguard their intellectual property rights enough or partake in patent infringement. IP non-compliance can impede investment decisions, deter novel ideas and result in expensive legal disputes.
Protecting intellectual property is vital for innovation in India’s pharmaceutical sector. However, challenges like patent infringement and complex regulations can stifle progress and investment in research.
7. Regulatory Compliance in Research and Development
Pharmaceutical firms must follow regulatory frameworks throughout the research and development (R&D) phase. Not following rules, giving wrong trial results, or not getting permission from participants can cause problems with following rules. Not following research rules can make it take longer to approve drugs, put patients at risk, and harm a company’s image.
Meeting regulatory standards during research is important in India. Delays and bureaucratic hurdles can slow down drug approval, while ethical concerns like proper consent are crucial for patient safety and industry credibility. Strengthening regulations and transparency is essential for ethical research practices in India.
With that, let’s explore strategies here to effectively manage compliance.
Strategies for Effective Compliance Management
Pharmaceutical compliance management is important for consumer safety. It helps in ensuring businesses follow a reliable industry standard. According to an Elsevier publication, increased compliance regulations reduce risk by 80%.
Forbes Technology Council reported that one Fortune 500 company received a $95 million fine. This fine is subject to compliance rules not being followed. This demonstrates that non-compliance incidents can have severe financial repercussions. It also erodes consumer confidence in an organisation.
Teams should focus on making compliance management simple and efficient to ensure success.
1. Integrated Compliance
Integrated compliance meets compliance requirements by integrating them into the business’s daily operations. In this approach, organisations prioritise meeting regulatory obligations and avoiding legal penalties. It is often seen as a more conventional approach to compliance.
An integrated compliance program includes rules and strategies in the pharmaceutical industry. It assures employees understand their legal duties and roles. This program helps employees follow regulations and standards and prevent violations and penalties. The system is designed to promote ethical behaviour and accountability within the organisation.
It contributes to the assurance that organisations fulfil their legal responsibilities. By including the rules in their daily operations, organisations can follow them. Moreover, the organisation can also save time and resources on compliance tasks.
Integrated compliance contributes to establishing a compliance culture throughout the organisation. Businesses can foster a common awareness of accountability among their employees. Emphasising compliance ensures adherence to regulatory requirements. It also helps integrate it into the organisation’s fundamental principles.
2. Understand the Landscape and Conduct a Thorough Risk Assessment
Most industries recognise regulatory standards. They use them to set the structural basis for strategy in the pharmaceutical industry.
Recognise potential failure points, characteristics, preventative measures and corrective actions based on industry expertise and research.
This principle doesn’t need rules. It’s based on understanding the business, customers, the main problem the product solves and other business factors.
3. Compliance Training
Only 29% of organisations continuously assess staff skills and compliance proficiencies.
Compliance training helps employees understand and follow an organisation’s laws, policies and procedures. It serves an essential function in:
-
Increased protection from legal liability
Compliance education is pivotal in protecting organisations. It disseminates pertinent laws, regulations and industry standards to employees. This mitigates the potential for expensive legal proceedings or penalties resulting from employee negligence or non-compliance.
-
Improved employee performance
Through instructional programs, employees can be more productive and better understand their responsibilities. So, customer experience, profitability and performance are all enhanced.
-
Elevated organisational culture
Compliance training fosters an atmosphere where all individuals know ethical principles. It helps managers and staff communicate and address issues before they become problems.
Leveraging JTB India’s Strategic Meetings Management Services for Compliance
Here’s how you can leverage our SMM services.
-
Comprehensive Compliance Integration
JTB India’s SMM services incorporate compliance considerations into each strategic management phase. We integrate compliance protocols throughout the agenda development. Venue selection processes are also ensured to ensure consistency with regulatory requirements.
-
Expert Guidance and Support
JTB India offers access to compliance specialists with extensive knowledge of regulatory standards in the pharmaceutical industry. These experts assist pharmaceutical companies in navigating complex compliance challenges. They also provide guidance and support throughout the meeting planning process.
-
Customised Compliance Solutions
JTB India knows compliance regulations differ according to attendee demographics and geographic location. Thus, they provide individualised compliance solutions tailored to each pharmaceutical client’s requirements. Through this, comprehensive and targeted compliance management can be maintained.
-
Technology-Driven Compliance Tools
JTB India optimises its compliance management process with the technology platform JTB Connect. Their technology-driven solutions streamline regulatory compliance duties. It also promotes effective management, encompassing automated documentation systems and real-time monitoring tools.
-
Ongoing Compliance Monitoring and Evaluation
Compliance monitoring is a continuous procedure performed by JTB India’s strategic meetings management services. In meetings, they address issues that arise and check compliance on an ongoing basis. Moreover, it helps identify potential areas for improvement. By being proactive, pharmaceutical companies ensure that standards are met throughout the lifecycle.
That’s not all. Here are some tips for you to implement.
Compliance Best Practices for the Pharmaceutical Industry
Here are the 4 best practices that you can implement:
1. Effective Risk Management Strategies in the Pharmaceutical Industry
Risk management is not a good practice in an industry where even minor errors can have significant and far-reaching consequences. Organisations should adopt proactive approaches to identify potential risks. They must address them before they escalate. This could involve:
- Regular risk assessments
- Development of suitable plans
- Maintaining constant attention to address emerging challenges
1. Knowledge of the Regulatory Landscape
Staying ahead of the ever-evolving regulatory landscape is key to maintaining a competitive edge in the pharmaceutical industry. Organisations must be aware of any modifications, additions or novel regulations that could impact their operations. Regular training and continuous education are vital in arming teams with the necessary knowledge. It helps to make informed decisions based on up-to-date information.
2. Quality Assurance and Control
Ensuring regulatory compliance is predicated on the maintenance of the highest quality products. Ensuring that products adhere to regulatory standards and inspire confidence in stakeholders and consumers is achieved through rigorous quality control measures and assurance processes.
3. Robust Documentation and Record-Keeping
Compliance with regulations is a need and a source of security and confidence. Accurate documentation in research and development, manufacturing and distribution are all phases of the pharmaceutical lifecycle that must be documented.
These records serve as evidence of compliance with established protocols. It promotes traceability by simplifying the process of identifying. It also resolves any potential problems, giving you the confidence that you are on the right track.
Conclusion
Regulatory compliance in pharmaceutical industry stands as a cornerstone for success, ensuring the safety, efficacy and ethical promotion of medicines and remedies. With the ever-evolving landscape of regulations and the potential risks associated with non-compliance, pharmaceutical companies must prioritise compliance in all operations.
Effective pharmaceutical meeting planning plays a crucial role in maintaining compliance standards, from meticulous content creation to seamless execution and post-event analysis. By leveraging JTB India’s Strategic Meetings Management services, pharmaceutical companies can navigate the complexities of compliance with confidence and precision.
JTB India’s SMM services offer comprehensive solutions tailored to the unique requirements of pharmaceutical meetings and events. From strategic planning to flawless execution, their expertise ensures alignment with objectives and delivers transformative meeting experiences. To maximise the benefits of strategic meetings administration and ensure compliance excellence, partner with JTB India.